Abstract
MYC, a well-known oncogene, is implicated in a majority of cancers, but there is little data on its role in myeloid malignancies. We recently described a novel internal tandem duplication of MYC gene (MYC-ITD) in a subset of patients with AML. As the prevalence of MYC aberrations have not been well documented in AML, we interrogated the genome, transcriptome and associated clinical and laboratory data from the largest cohort of pediatric AML patients to define the prevalence, frequency of co-occurring cytomolecular alterations and outcomes associated with MYC variants.
Mutation Profiling: Diagnostic specimens from 1,806 patients with AML (age 0-29 years) enrolled on COG Phase III trials were retrospectively interrogated using contemporary NGS techniques or a PCR based "Tandem Repeat" assay for MYC-ITD. Conventional karyotyping and mutational analysis, including NGS, were used to determine additional cytogenetic and molecular abnormalities. Of the 1,806 patients studied, MYC aberrations were identified in 58 patients (3.2%) with 50% (N=29) harboring MYC-ITD, 46.5% (N=27) with a SNV or indel (MYCSNV/indel) and 2 patients (3.5%) with dual MYC-ITD and SNV. For MYC-ITD, tandem duplications occurred in exon 3 (Max binding domain) with ITD lengths ranging from 252-588 bp. The majority of MYCSNV/indel (77%) were identified at the P72-P78 hotspot with 33.3% (N=9) harboring the P74>L mutation and 18.5% (N=5) having the T73>N mutation.
Functional Studies: Given the novelty of the ITD variant, we initially characterized the functional properties of MYC-ITD in vitro. Similar to wild-type (MYCWT), MYC-ITD is localized to the nucleus and requires Max to bind to specific DNA sequences (E-boxes). However, in contrast to MYCWT, MYC-ITD fails to recognize sequences containing a single E-box in vitro, but exhibits higher affinity toward sequences with two or more E-boxes. MYC-ITD is consistently more efficient in activating promoters with multiple E-boxes than MYCWT. Thus, MYC-ITD may possess different DNA-binding and protein-interacting properties, potentially leading to altered transcriptional regulation and biological output. Given the alterations in DNA binding properties, we evaluated the functional consequences of MYC-ITD in our cord blood stem cell/endothelial cell (EC) co-culture system. Normal cord blood stem cells were transduced with MYC-ITD vs. MYCWT and grown in an EC co-culture system. MYC-ITD transduced cord blood stem cells showed significantly higher proliferation compared to MYCWT or control GFP-transduced cells with proliferation advantage of 37-and 45-fold after 14 and 21 days in co-culture, respectively (p<0.001).
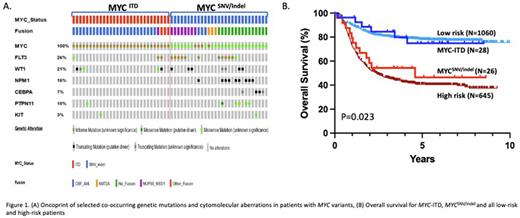
Clinical Impact: Analysis of cooperating cytomolecular lesions revealed a striking difference between MYC-ITD and MYCSNV/indel. MYC-ITD was highly enriched in core-binding factor (CBF) AML found in 86% (N=25)[(N=18, 62%) with inv(16) and (N=7, 24%) with t(8;21)] (Figure 1A), vs. 26% in the MYCWT cohort and had a paucity of somatic mutations. Conversely, MYCSNV/indel was enriched in NUP98-NSD1 fusions (N=7, 27%), FLT3-ITD (N=10, 38%) and NPM1 mutations (N=9, 33%) (Figure 1A), which was significantly higher than the WT cohort. Patients with MYC-ITD had a morphologic CR rate after course 1 of 92.3% vs. 80% (p=0.249) in patients with MYCSNV/indel with a corresponding relapse rate of 24% and 52% (p=0.046). Lower CR and higher relapse reflects the higher proportion of patients with high-risk lesions (FLT3-ITD, NUP98-NSD1 and WT1) in MYCSNV/indel and favorable CBF in MYCITD. Those with MYC-ITD and MYCSNV/indel had a 5-year event free survival (EFS) of 70% vs. 38.5%, respectively (p=0.018) with a corresponding overall survival (OS) of 76% and 47% (p=0.023) similar to patients with favorable or high risk disease (Figure 1B).
Conclusion: Here we define MYC aberrations as a new class of pathogenic variants in childhood AML. We demonstrate functional and clinical characterization of a novel MYC-ITD and contrast this novel variant to that of established pathogenic MYCSNV/indel. We show the high prevalence of co-occurring core-binding factor fusions with MYC-ITD, and high frequency of co-occurring NUP98-NSD1, FLT3-ITD and NPM1 mutations in MYCSNV/indel. Patients with MYC-ITD have superior clinical outcomes while patients with MYCSNV/indel have clinical outcomes similar to high-risk patients, likely due to the associations with the disease associated fusions.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.